Minimum case numbers in Germany
Welcome again! We are extending the short excursion to quality assurance today with a look at minimum case numbers in Germany. In
German Market Access – Simplified
Everything you need to know in one place, explained in simple terms
Welcome again! We are extending the short excursion to quality assurance today with a look at minimum case numbers in Germany. In
Welcome to the second post in the quality assurance series, focusing on the hot topic of ATMPs! Next week, I’ll be focusing
Hi again, welcome to the series in quality assurance initiatives, starting with disease management programmes in Germany. This post will cover: Next
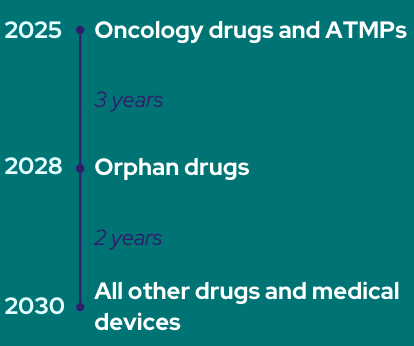
Welcome to the last post in the HTA series, focusing on the changes to HTA and early benefit assessments in Germany in
Welcome! In this post, I am going through the steps on how the G-BA chooses the appropriate comparative therapy (ACT). I conclude