Which endpoints are important to the G-BA and IQWiG?
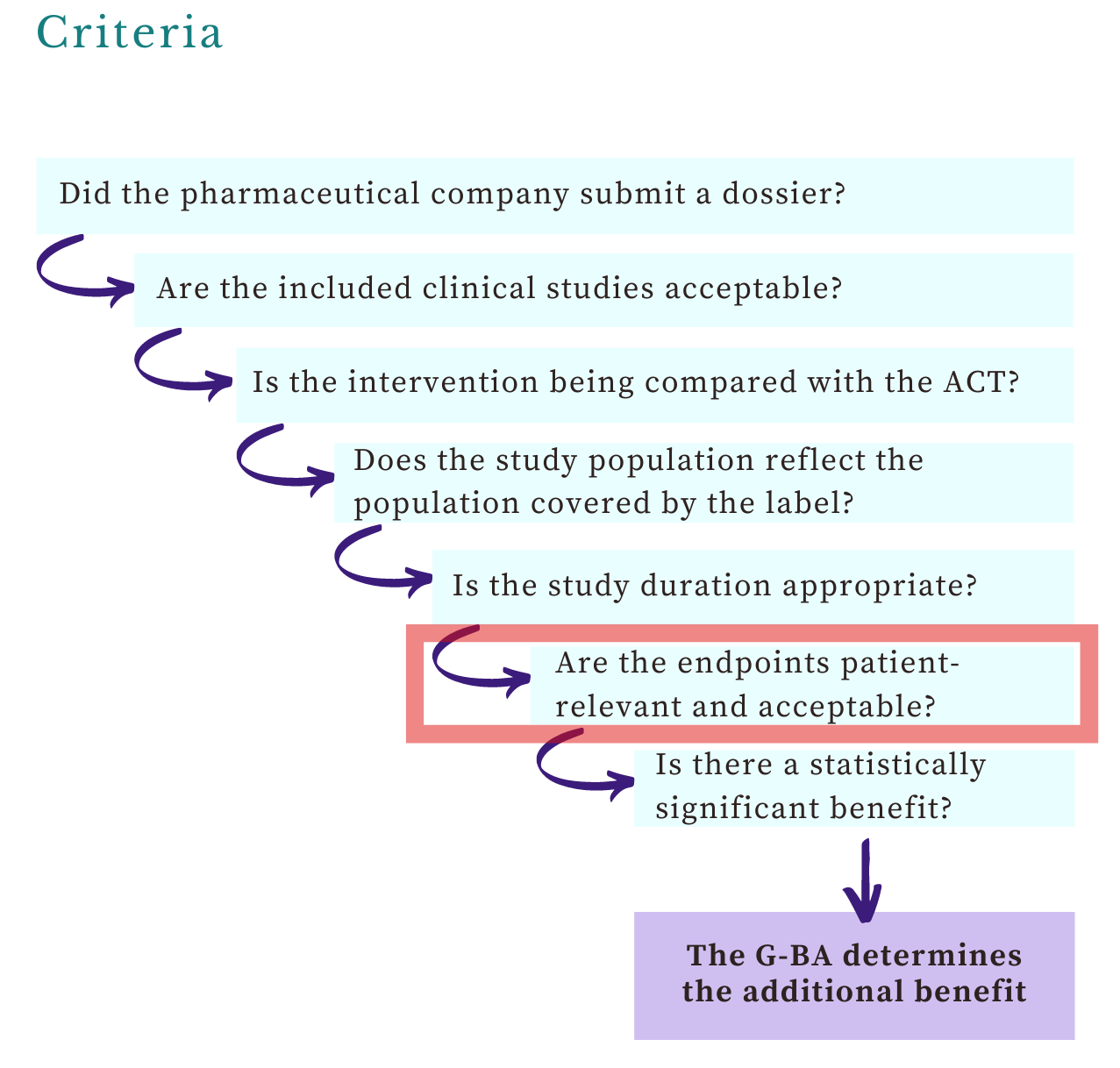
Welcome, this week’s post on the G-BA’s views on endpoints is the second article in the benefit assessments series. You’ll learn about:
German Market Access – Simplified
Everything you need to know in one place, explained in simple terms

Welcome, this week’s post on the G-BA’s views on endpoints is the second article in the benefit assessments series. You’ll learn about:
This is the first article in a series about maybe the most important topic influencing market access in Germany: HTAs or early
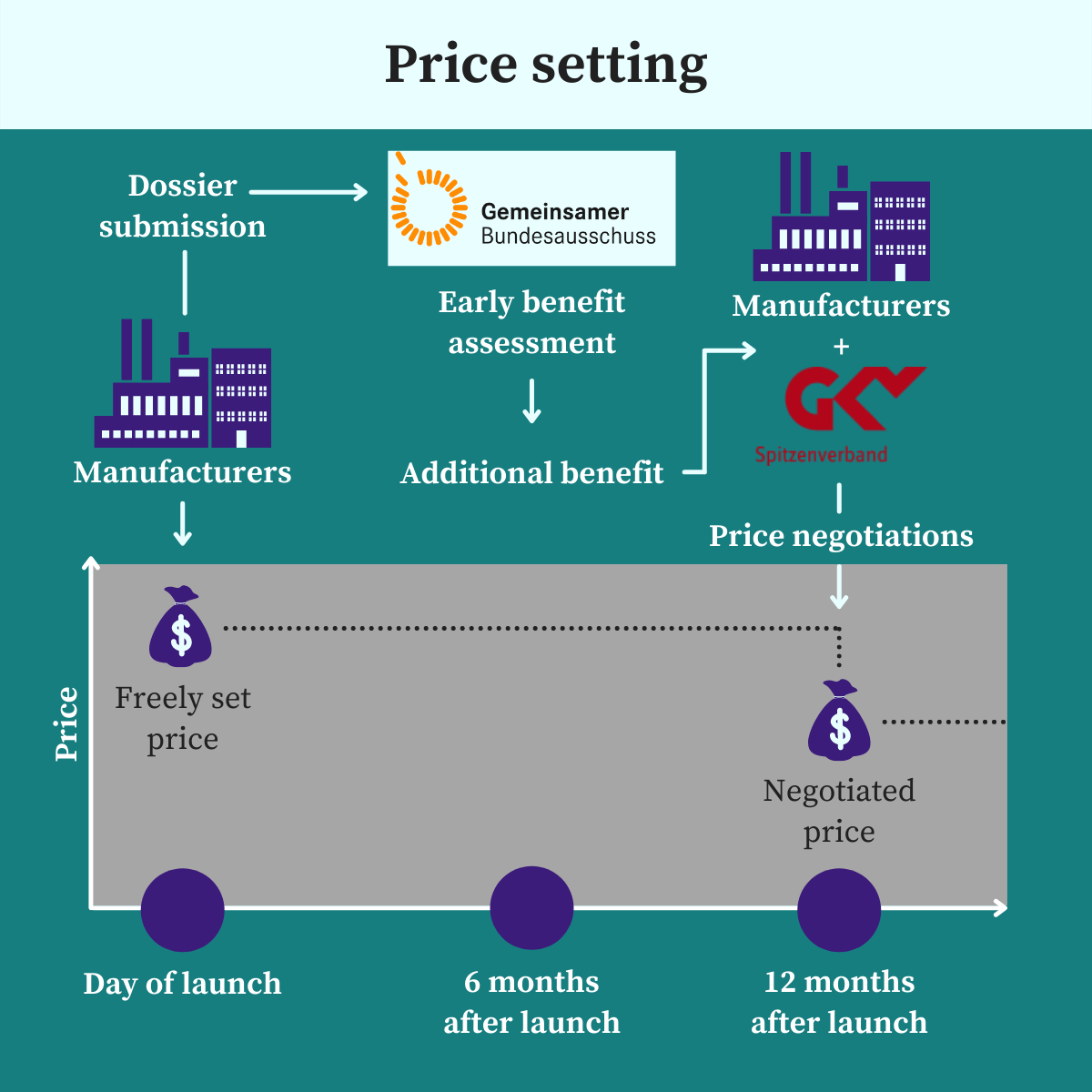
Currently, in Germany, free pricing applies in the first year. This means, at the time of launch in the German market, pharmaceutical companies are free to set the price for their product with a new chemical entity (NCE). This price is then valid for the first 12 months from the day of launch.

On 6 December 2021, Prof. Dr. Karl Lauterbach was announced as the new German health minister. This might mean quite a lot

The new government in Germany is formed as a coalition of the three parties, SDP, FDP and the Green Party. At the