German Health minister presents new draft of act to stabilise SHI fund finances
In March 2022, Lauterbach shared a non-authorized draft of his reform plans to stabilise the SHI funds finances. However, this draft version
German Market Access – Simplified
Everything you need to know in one place, explained in simple terms
In March 2022, Lauterbach shared a non-authorized draft of his reform plans to stabilise the SHI funds finances. However, this draft version
Hello! In this week’s post, I am describing the G-BA’s initiative to promote innovation in the German health care system and clinical
In this post, I’ll summarise the key points on the real-world evidence data collection in Germany, that the G-BA can request since
Hi again, welcome to the series in quality assurance initiatives, starting with disease management programmes in Germany. This post will cover: Next
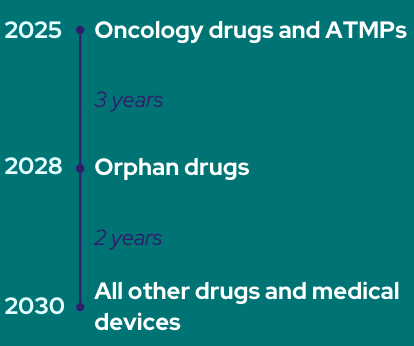
Welcome to the last post in the HTA series, focusing on the changes to HTA and early benefit assessments in Germany in