How does the G-BA pick the appropriate comparator?
Welcome! In this post, I am going through the steps on how the G-BA chooses the appropriate comparative therapy (ACT). I conclude
German Market Access – Simplified
Everything you need to know in one place, explained in simple terms
Welcome! In this post, I am going through the steps on how the G-BA chooses the appropriate comparative therapy (ACT). I conclude
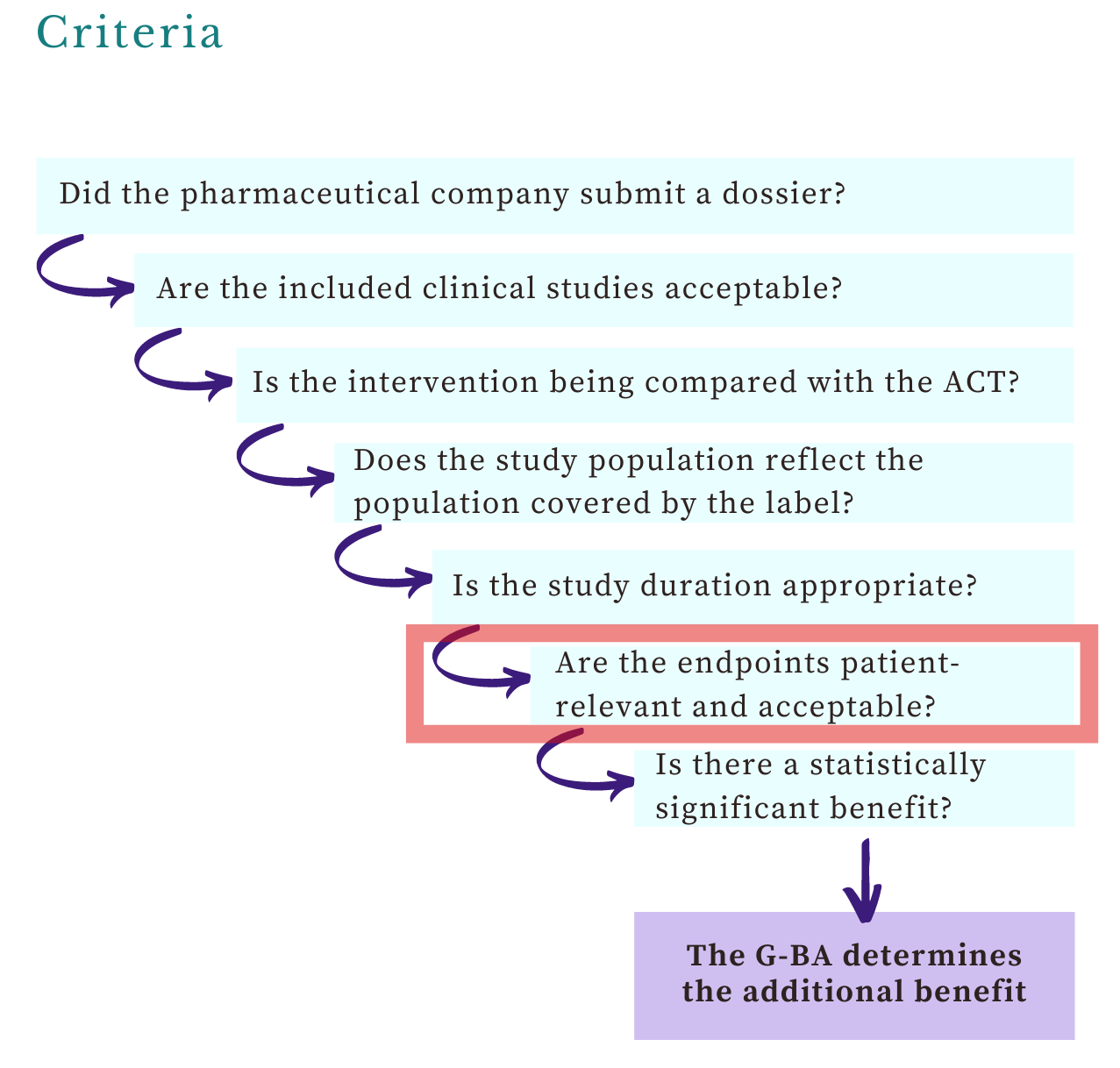
Welcome, this week’s post on the G-BA’s views on endpoints is the second article in the benefit assessments series. You’ll learn about:
This is the first article in a series about maybe the most important topic influencing market access in Germany: HTAs or early

Manufacturers of drugs or medical devices can apply for a consultation meeting with the G-BA before submitting a dossier. This consultation offers

On 6 December 2021, Prof. Dr. Karl Lauterbach was announced as the new German health minister. This might mean quite a lot