Can the expected medical device shortages in Germany be stopped?
Serious medical device shortages in Germany have been observed – and more are expected – as a consequence of the new EU
German Market Access – Simplified
Everything you need to know in one place, explained in simple terms
Serious medical device shortages in Germany have been observed – and more are expected – as a consequence of the new EU
Since 2020, clinicians can prescribe – and SHI funds will reimburse – medical apps and digital health treatments, the so-called “DiGAs”. The BfArM assesses these DiGAs
Following on from the EU regulation for medical devices (MDR) that came into force over one year ago, this week I am
Hi again! This week I am switching it up a bit, and look at medical devices, particularly the EU regulation for medical
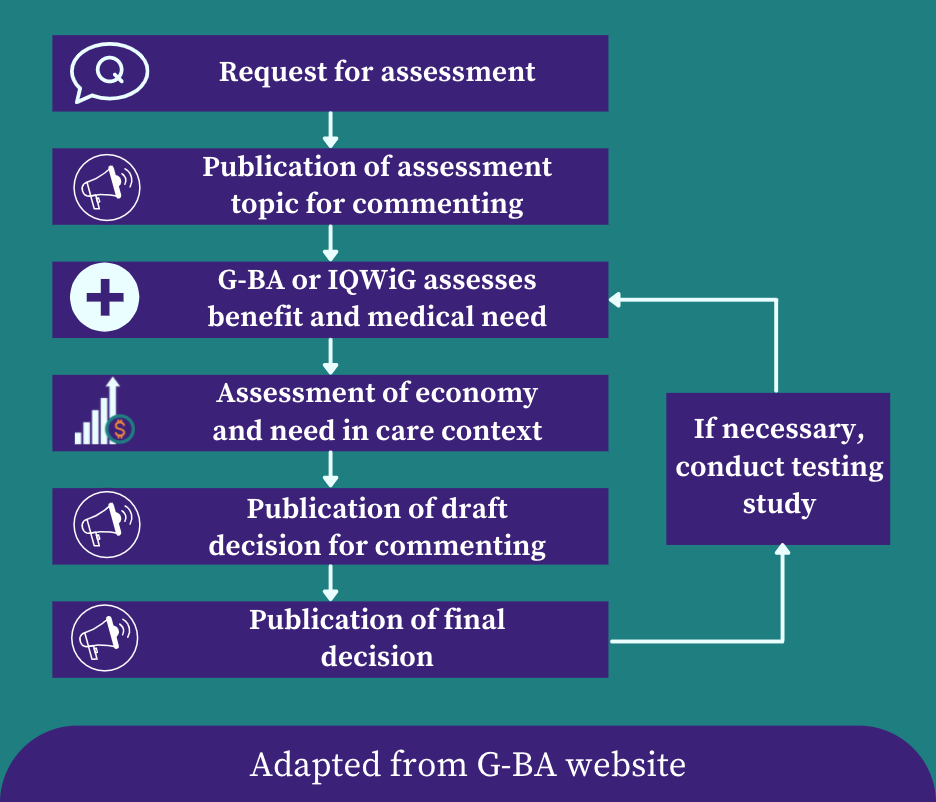
Hello again, and welcome to the second post in the 3-part series on reimbursement. Last week, we focused on the reimbursement of